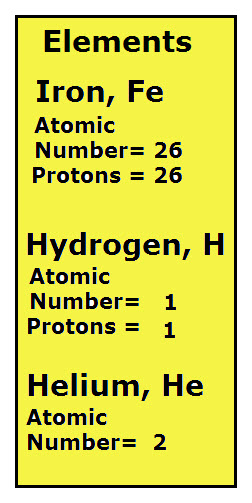
Atomic numbers are the ID numbers for all known elements. Each element has its own specific atomic number. For example, in the list of elements note that each has its own atomic number. The atomic number for an element also provides formation an element’s atomic particles.
Protons
Protons are the positive particles in an atom. The atomic number of an element is equal to the number of protons the element’s atoms have. Therefore, since the atomic number of Iron, Fe, is 26, all iron atoms have 26 protons.
Helium, He, has an atomic number of 2, can you predict the number of protons in each atom of He?
An element’s atomic number equals the number of protons in each atom of the element. If you predicted that He, with an atomic number of 2 has 2 protons in each of its elements, then your prediction is correct.
Electrons
Electrons are negative particles in atoms. The number of electrons in each atom of an element is equal to the elements atomic number. Thus, an elements atomic number is equal to the number of protons and electrons in an element’s atoms. For example, Iron, Fe, has an atomic number of 26, thus the atoms of this element has 26 protons and 26 electrons.
The list of elements compares the atomic number and protons for three elements, iron, hydrogen and helium. Notice that the atomic numbers are different and that the protons for each element is the same as the atomic number.
Can you predict the number of electrons for the atoms of Fe, H, and He?
Remember that the atomic number is equal to the same number of protons and electrons for each element. If you predicted that Fe has 26 electrons, H has 1 electron, and He has 2 electrons, then your predictions is correct.
Each of the 101 chemistry experiments has a purpose, list of materials, step-by-step instructions and illustrations, expected results, and a science explanation in understandable terms.
(paid link)
Big Book of Science Experiments
A book of fun informative experiments about astronomy, biology, chemistry, earth science, and physics.
(Paid Link)