Indicators
Acid Indicators are materials that change color in an acid solution.
Base Indicators are materials that change color in a base solution.
Acid/Base Indicators are materials that have one color when mixed with an acid and another color when mixed with a base.
Red Cabbage Juice is an acid/base Indicator. The juice has a purple color that changes to yellow in a base and to red in an acid.
Acids are materials that have certain properties in common.
- One property is how acids affect the color of different indicators.
Acids turn BLUE LITMUS INDICATOR RED.
Strong Acids turn red cabbage indicator Red
Acids are compounds that donate a proton in a chemical reaction.
The proton donated by most acids is the hydrogen ion, H +.
Bases (also called alkalies) are materials that have properties opposite those of acids.
- One property is how bases affect the color of different indicator.
Bases turn RED LITMUS INDICATOR BLUE.
Bases turn red cabbage indicator yellow.
Bases accept protons in a chemical reaction.
PREPARE RED CABBAGE INDICATOR
1.  Instruction for preparing red cabbage indicator can be found HERE Or Click on the Picture of the Red Cabbage.
Instruction for preparing red cabbage indicator can be found HERE Or Click on the Picture of the Red Cabbage.
Keep the cabbage indicator stored in a sealed jar in the refrigerator. This indicator sours when warm and spells awful.
2. Use the cabbage indicator to test different substances. Do this by pouring a small amount of the cabbage indicator into a see-through plastic glass. Note, if the cabbage indicator is very concentrated, add DISTILLED WATER to dilute it. You want the purple color to be intense, but still be able to see through the colored liquid.
Using an eye dropper, add drops of the testing liquid to the indicator, one drop at a time. Swirl or stir the liquid so that the testing liquid is thoroughly mixed.
Continue adding the testing liquid until there is no further color change.
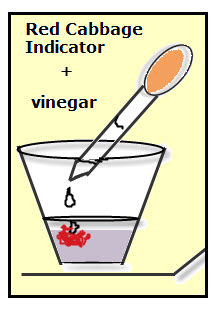 3. To test solids, crush the solid until it is a fine powder, and then add a small amount of the powder to the indicator. Stir or swirl to mix. Continue adding the powder until there are no further color changes.
3. To test solids, crush the solid until it is a fine powder, and then add a small amount of the powder to the indicator. Stir or swirl to mix. Continue adding the powder until there are no further color changes.
 |
Chemistry For Every Kid |

