What is Cohesion and How does it Relate to Surface Tension
Cohesion is the attraction that like- molecules have for each other.
Water molecules have an attraction for each other. This attraction is called cohesion.
Surface Tension
Surface tension is the skin-like surface of a liquid due to the cohesive forces between the liquid molecules. The diagram below shows an imagined magnified view of the surface of water, the molecules of liquid at the surface do not have other like- molecules above them. Thus, the surface molecules are pulled in all directions by the cohesion (forces between like-molecules) between the water molecules—except upward. Thus the surface molecules have more forces pulling them toward the wa
ter molecules below and on each side, while water molecules below the surface are pulled in all directions. The cohesive forces acting on surface liquid molecules is referred to as surface tension.
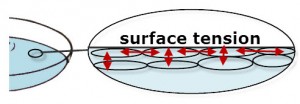
While not meant to be an exact replica, the elliptical shapes represent water molecules. Notice the forces between the molecules represented by the red arrows.
Surface tension causes the surface of a liquid to pull together forming the smallest surface area possible.When a liquid is in a container, such as water in a glass, the liquid is also attracted to the molecules making up the container. Thus, while cohesion tends to pull water molecules into a
sphere shape, the force of attraction between water and the glass keeps the water against the inside surface of the glass.
The force of attraction between unlike-molecules is called adhesion.
Often it is said that water is trying to form the shape with the smallest surface area, which is a sphere (ball-shaped). I am sure that I have said this and may even have it in one of my books. But, I am becoming more aware of statements that give non-living objects (and even plants) the ability to think and reason. With this thought in mind, water is not “trying to do anything.” Instead, because of the natural cohesive forces between water molecules and the fact that surface molecules have a surface tension, without a container, water forms spheres. The perfect example of this are water drops in space craft at zero gravity.
I have been at zero gravity and did see liquid spheres form, only it was the gastric juice from my stomach that had not been captured by the barf bag. They floated –perfectly spherical–for a few seconds. Then, SPLAT! The NASA Vomit Comet (airplane) I was in started to ascend, thus gravity pulled the spheres down and into the fabric of my flight suit.
Notice how the dripping water in the photo above begins to form a sphere shape. When this section of the water is pulled free by gravity, will the water retain a spherical shape? Or does it form a tear-drop shape that is often used to represent rain drops?
Think about it: As the water drop falls it hits into the gas molecules in air as well as dust particles. In other words, the sphere that leaves the end of the stream of water like anything falling through air, has an upward force on its leading edge.
Need another clue? Put a drop of water on something that is water resistant, such as a piece of wax paper. Look at the drop from the side. Notice how flat the bottom of the drop is and ho
w round it is above the paper.
Yes, raindrops are round with a slightly flattened bottom. This makes me think of a basketball that needs air. Sit it on the floor and its bottom is flat.
Challenge: Now that you have an understanding of surface tension. Can you explain why it is harder to move an object thrugh the surface of water than it is to move the same object when completely submersed in the water.
 |
For lots of fun science information and investigation. |
