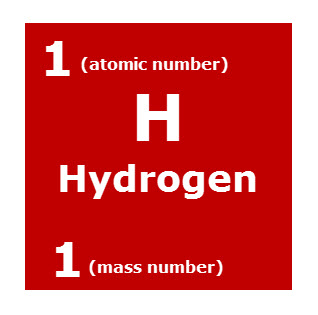
What Do the Numbers on the Periodic Table Mean?
The red box is an enlargement of the hydrogen box from the periodic table of elements. The four identified parts for the hydrogen box shown are:
1. H, element symbol: All elements have a symbol. Some symbols have one letter and some have two letters. The first letter of all element symbols are capitalized and if there is a second letter it is lower case.
For more information, see element symbols for natural elements.
See Symbols for Synthetic Elements . This activity also has information about the temporary symbols used for new elements until they have an official name.
2. Hydrogen, element name: The name, Hydrogen, is capitalized in the box, but elements are not proper names and do not have to be capitalized. Thus, Hydrogen or hydrogen is correct.
3. Atomic Number: This number is generally listed at the top of the box. The atomic number is a very important code. It tells you two things about the element:
(1) The atomic number is equal to the number of protons in the nucleus of every hydrogen atom. Since hydrogen has an atomic number of 1, every atom of hydrogen has one proton in its nucleus.
(2) The atomic number is equal to the number of electrons outside the nucleus of each of the element’s atoms. For hydrogen, with an atomic number of 1, every hydrogen atom has 1 electron outside its nucleus.
4. Mass Number: The mass number is generally at the bottom of the box. The mass number equals the total number of nucleons (protons + neutrons) in the nucleus of each of the element’s atoms.
Mass Number = # P + # N ( P is the symbol for protons, and N is the symbol for neutrons).
The difference between the atomic mass and the atomic number equals the number of neutrons in the nucleus of the element’s atoms.
For Hydrogen: The number of neutrons = 1 – 1 = 0
Atomic Diagram for Hydrogen
Atomic diagrams contains a circle to represent the nucleus of the atom with semicircles outside the nucleus to represent the energy levels in which electrons are found.
Note that the diagram for hydrogen has a nucleus with one energy level. Inside the nucleus is one proton, 1P. Outside the nucleus in the one energy level is one electron, 1 e –. Since hydrogen atoms have no neutrons, none are represented.
Big Book of Science Experiments
A book of fun informative experiments about astronomy, biology, chemistry, earth science, and physics.
(Paid Link)