 What are Polymers?
What are Polymers?
How are Polymers Made?
How are Polymers Broken?
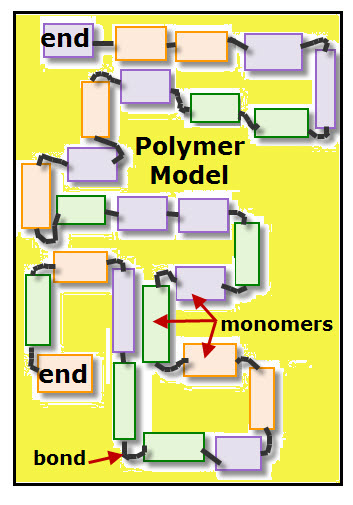
Polymers are macromolecules, which means very large molecules. Each polymer is a chain of identical or similar links called monomers.
How are monomers linked together to form polymers?
The process of linking monomers is known as condensation, or dehydration synthesis. Remember that the term synthesis always indicates that something is being made–building blocks of chemicals are being linked together by electrical forces called bonds.
Covalent b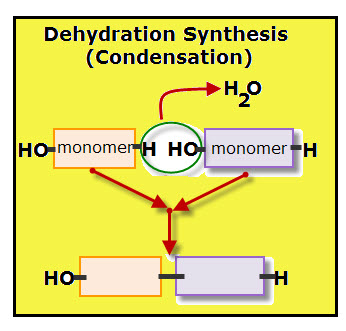 onds are formed between two atoms that share electrons.
onds are formed between two atoms that share electrons.
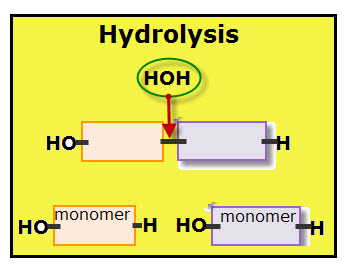
For each monomer added to the polymer chain, one molecule of water is removed. Each molecule of water is made of two hydrogen atoms and one oxygen atom.
The diagram shows the dehydration of when two monomers link together. Notice that each monomer contributes part of the water molecule. The orange colored monomer contributes one hydrogen (H) while the purple colored monomer contributes one hydroxyl group (OH). Together the hydrogen and hydroxyl group form one molecule of water.
A covalent bond links the two monomers.
Anabolism are the pathways that connect small units forming larger molecules. This requires energy. Names for the building of polymers are: condensation, dehydration synthesis and polymerization.
How are Polymers Disassembled to Separate Monomers?
Hydrolysis is the chemical reaction that disassembles connected monomers. It other words, hydrolysis is a catabolic reaction, which are the pathways that break down large molecules into small units with the release of energy.
 |
A+ Projects in Biology |