 Atoms are the smallest particle of an element that retains the characteristics of the element.
Atoms are the smallest particle of an element that retains the characteristics of the element.
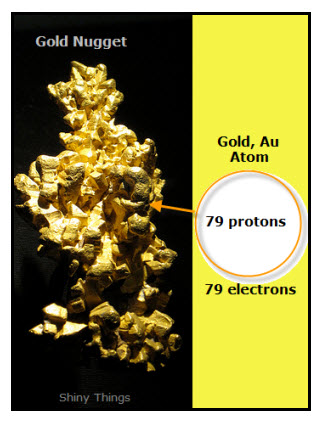
For Example: The gold nugget in the diagram is made up of individual gold atoms.
Gold Atom Diagram
The circle represents the nucleus of a gold atom. Outside the nucleus of the gold atom are 79 electrons (negative charges). Electrons move around the nucleus.
Inside the nucleus of the gold atom are 79 protons (positive charges).Only carbon atoms have 79 protons.
I repeat: ONLY GOLD ATOMS have 79 protons.
Atomic Number:
Every element has a different atomic number. The atomic number of an element is the number of protons that each atom of that element has. The atomic number of Gold is 79.
Every gold atom has 79 protons. Only gold atoms have 79 protons.
Each gold atom has 79 protons in its nucleus. Have you noticed that the number of protons for a gold atom is equal to the number of electrons that a gold atom has?
FACT: An atom has an equal number of protons and electrons. All gold atoms have the same number of protons and electrons. But all gold atoms are not exactly alike.
 Mass Number
Mass Number
The mass number of an atom is the sum of the protons and neutrons in the nucleus of the atom.
Neutron: A particle found inside the nucleus of an atom. This particle does not have a charge.
The Mass Number for the gold atom in the diagram is 79 P + 118 N = 197
Isotopes
Isotopes: Atoms of the same element that have different atomic masses.
Isotopes: Atoms of the same element that have the same number of protons, but a different number of neutrons.
Isotopes can be identified by writing their name or symbol and their mass number. For example:
Gold-197 or Au-197. This is the only natural isotope of gold. The mass number of this isotope is given after the elements name. The diagram for Au-197 contains 79 protons and 118 neutrons
Challenge:
1. What is the name of the gold isotope shown in the diagram to the left?
Answer:
Think!
1. You know it is a gold isotope because it has 79 protons.
2. The mass number of any atom is equal to the number of protons plus the number of neutrons in the atom’s nucleus.
3. The mass number for the diagram is: 79 protons + 119 neutrons = 198
4. The name of a specific isotope of an element is the:
elements name + mass number (gold-198)
symbol of the element + mass number (Au-198)
 |
Sticky, Gloppy, Wacky, and Wonderful Experiments |