Who Developed the Periodic Table of Elements?
The periodic table of elements was designed independently about the same time by two different scientists. Credit is given to Dmitri Mendeleev, a professor of chemistry in St. Petersburg, Russia because he published the first version of the table in 1869. Julius Lothar Meyer (1830-1895), a chemistry professor in Tuebingen, Germany, prepared a table but didn’t published it until 1870. Mendeleev’s table differed from Meyer’s in that he left spaces and predicted that elements with certain properties would be discovered. This turned out to be true, thus Mendeleev’s table was widely accepted and the modern periodic table is based on Mendeleev’s table.
Development of the
Periodic Table of Elements
As more and more elements were discovered, chemists saw similarities in them. They noticed that groups of elements had similar properties. Over time, scientists searched for ways to organize elements and many different systems were used.
In 1869 the Russian scientist Dmitri Mendeleev (1834-1907) developed a system that the modern arrangement of the periodic table is based on.
Mendeleev’s arrangement of the elements was in order of increasing atomic masses.
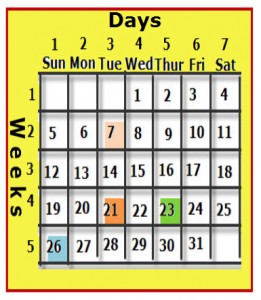
He made a table of the elements similar to that of a monthly calendar, which has columns (vertical groups) and rows (horizontal groups).
On a calendar, the rows represent a specific period of time, which is seven days also called a week. The columns represent a specific day of the week, and each day is given a name. On the calendar shown, day- 1 or the first day of every week is Sunday. No matter which day of the week you select, seven days later falls on the same day of the week. In other words, if you select Wednesday the 1st, seven days later will be Wednesday the 8th.
Mendeeleev’s Repeating Pattern
Mendeleev found that his arrangement of the elements, like the calendar, had similar patterns of repeated properties. Things with a repeating pattern are said to be periodic. The time of one week is the repeating pattern for a calendar. Mendeleev’s periodic table of element had repeated patterns of physical and chemical properties.
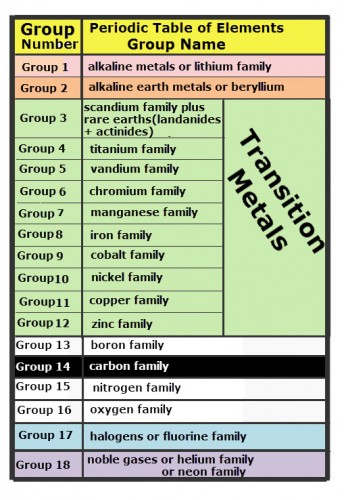
Like a calendar, Mendeleev’s arrangement of the elements formed a table with columns representing elements that are alike. These vertical columns are called groups. The table of groups on the periodic table of elements and the names for each group is shown.
In order for Mendeleev to arrange the elements by their properties, he had to leave some blank spaces in his table. Mendeleev’s periodic table was based on the idea that the physical and chemical properties of the elements are periodic functions of their atomic masses.
But, listing the elements in order by their mass resulted in some element not properly fitting in Mendeleev’s table. Mendeleev assumed that his table was correct and that the error was in the mass of the “misfit” elements.
In 1913, experiments performed by the English scientist Henry Mosely (188701915) showed that physical and chemical properties of elements are periodic functions of their atomic numbers (number of protons in an atom of an element). This solved the problem of the “misfit” elements.
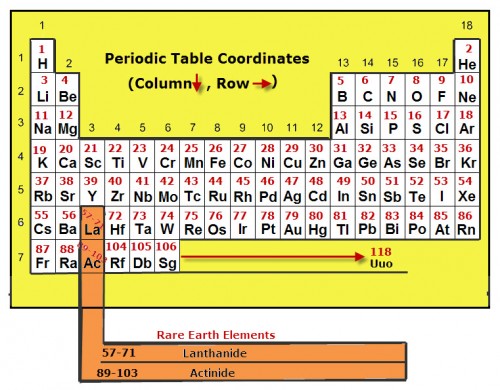
The modern periodic table of elements is arranged by the atomic numbers of each element. The periodic table shown here is very basic, showing only the symbols and atomic numbers for each element. The group numbers as well as the row numbers are also labeled.
Big Book of Science Experiments
A book of fun informative experiments about astronomy, biology, chemistry, earth science, and physics.
(Paid Link)